Advertisement of Patanjali’s corona medicine is prohibits, Acharya Balakrishna sent reply
As soon as Baba Ramdev launched the medicine on Tuesday with the claim of completely correcting the corona in seven days, the Ayush ministry swung into action. After this, the Ministry of AYUSH immediately asked Patanjali to stop advertisements promoting the drug. The ministry made it clear that if the drug advertisement continues after this, legal action will be taken against it. A senior AYUSH ministry official said that Patanjali has not given any information about the development and trial of any such drug to the ministry.
He said that with the permission of the ministry, many Ayurvedic medicines are being tried in the treatment of corona, but they do not include the medicine of Patanjali. The senior official said that when the whole world is struggling to find a cure for corona and there is no way out. In such a situation, the claim of treatment with any medicine without scientific evidence can prove to be dangerous and crores of people can get caught in the trap of this misleading propaganda. That is why, with an immediate ban on advertisements promoting this drug, Patanjali has been asked to give details of the elements used in coronil medicine as soon as possible.
Patanjali will also have to tell which hospitals and how many patients have been tried on this drug. It is necessary to register the drug in the Clinical Trial Registry of India (CTRI) to start the trial. Patanjali has been asked to provide complete data of the results of the trial along with the registration of CTRI.
The senior official said that this drug will be allowed to be used in the treatment of corona after thorough investigation and facts are found correct. Actually, there is no need to get permission from the Ministry of AYUSH before developing and selling Ayurvedic medicine. But it is mandatory to get permission from the licensing authority of the state where the drug is being produced. The Ministry of AYUSH has asked the State Licensing Authority of Uttarakhand to give a copy of the approval and license given to this drug.
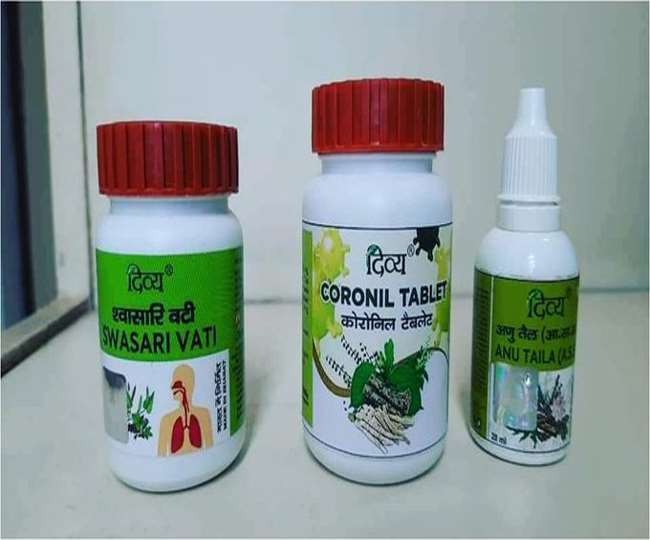
Patanjali’s drug licensed as an immunity booster
Patanjali’s drug Coronil has been issued a license by the Uttarakhand Ayush Department. The Ministry of AYUSH has sought information about this from the department. The licensing officer of the department, Dr. Yatendra Singh Rawat, says that the license has been issued on June 12 in the name of Divya Pharmacy. The license mentions two different amounts of inhaled medicine, including coronil vati, as immunity boosters. Now the company will be issued a notice asking on what basis it is prescribing immunity booster medicines as Covid-19.
The misunderstanding of the Ministry of AYUSH on Patanjali’s medicine is removed: Balakrishna
Acharya Balakrishna’s statement has come on the matter of banning the advertisement of Patanjali’s medicine. Acharya Balakrishna is the General Secretary of Patanjali Yogpeeth. He tweeted, ‘The central government has been promoting Ayurveda. Any misconceptions that the AYUSH ministry had about Patanjali’s medicine have been dispelled. Patanjali has met all official standards of randomized placebo controlled clinical trials. We have given all its information to the Ministry of AYUSH, now there is no doubt anywhere.
Patanjali used corona virus medicine
Let us tell you that Yoga Guru Swami Ramdev launched the corona virus drug Coronil on the market on Tuesday and claimed that this drug, made after intensive study and research of herbs from Ayurveda method, is benefiting 100 percent patients. Talking to reporters at the Patanjali Yogpeeth here, Baba Ramdev said that Patanjali is the first Ayurvedic institute in the world to market Corona epidemic medicine with authenticity after extensive study and research of herbs.
He said that this medicine is benefiting 100 percent patients. He said that a controlled clinical trial was conducted on 100 patients, in which 69 percent of patients were cured within three days and 100 percent within four days and their test report turned positive from positive.



